From Soybeans to Tofu: The Underlying Chemistry
Summary
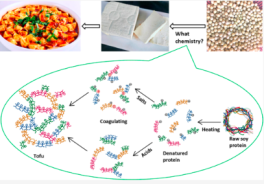
Tofu, a traditional Chinese food, is widely consumed around the world, yet the chemistry behind its production is often overlooked. This study presents a simple demonstration designed for lower-level undergraduate courses in organic chemistry or biochemistry to elucidate the key principles involved in the tofu-making process. The research highlights the role of heat treatment in denaturing soy proteins, which facilitates coagulation when coagulants such as calcium gluconate, zinc gluconate, and calcium lactate are added. Acidic coagulants, including white vinegar and citrus juices, also induce coagulation due to their impact on the isoelectric point of soy proteins. The study provides a detailed analysis of the curdling mechanism based on experimental data and previous research, making it an effective educational tool for both laboratory and at-home learning environments.
Summary:
Authors/Contributors: Bingxing Wang, Qi Wang, Bingli Wang, Songlin Wang, Yongcai Zhang, and Donglin Zhao.
Citation: Wang, B., Wang, Q., Wang, B., Wang, S., Zhang, Y., & Zhao, D. (2023). From Soybeans to Tofu: The Underlying Chemistry. Journal of Chemical Education, 100, 3724–3730. https://doi.org/10.1021/acs.jchemed.3c00096
Summary:
Authors/Contributors: Bingxing Wang, Qi Wang, Bingli Wang, Songlin Wang, Yongcai Zhang, and Donglin Zhao.
Citation: Wang, B., Wang, Q., Wang, B., Wang, S., Zhang, Y., & Zhao, D. (2023). From Soybeans to Tofu: The Underlying Chemistry. Journal of Chemical Education, 100, 3724–3730. https://doi.org/10.1021/acs.jchemed.3c00096
Safety Precautions, Hazards, and Risk Assessment
N/A
Teacher Recommendations or Piloting Data (if available)
N/A
Link to external